Generic Drugs: What They Are, How They Work, and When to Watch for Changes
When you hear generic drugs, brand-name medications sold under their chemical name after the patent expires. Also known as generic medication, they contain the same active ingredients, work the same way, and are held to the same FDA standards as their brand-name counterparts. For most people, switching to a generic is simple, safe, and saves hundreds a year. But for some, even tiny differences in fillers, coatings, or absorption rates can make a noticeable difference—especially with drugs that have a narrow therapeutic window, like thyroid meds, seizure drugs, or blood thinners.
That’s why therapeutic equivalence, the official rating that says a generic performs the same as the brand matters. Not all generics are created equal in practice, even if they’re rated AB1 by the FDA. Your body’s drug metabolism, how your liver and enzymes break down and process medication can react differently to slight variations in how the drug is released. If you’re on a medication where even a 5% change in blood levels could cause problems—like warfarin, lithium, or cyclosporine—you need to pay attention. Symptoms like new dizziness, mood shifts, unexplained fatigue, or worsening symptoms after a switch aren’t just "in your head." They’re signals.
Switching to generics isn’t about cutting corners—it’s about smart, informed choices. Many people never notice a difference. Others find their blood pressure stabilizes better, their seizures become less frequent, or their mood improves after switching from one generic to another. That’s because not all generics use the same inactive ingredients, and your body might respond better to one formulation over another. That’s why monitoring your health after a switch isn’t optional for some—it’s essential. And if something feels off, reporting it to the FDA isn’t complaining. It’s helping improve safety for everyone.
The posts below cover real situations where generics made a difference—or caused unexpected issues. You’ll find guides on what to watch for after switching, how genetic testing can predict your reaction, why some drugs need extra caution, and how to talk to your doctor about alternatives. Whether you’re on a tight budget, managing a chronic condition, or just trying to understand why your meds feel different, this collection gives you the facts—not the fluff.

Medicare Part D Economics: How Programs Use Generics to Cut Costs
Medicare Part D saves billions by steering beneficiaries toward generic drugs through tiered formularies, low copays, and financial incentives. Learn how the system works, where savings happen, and how to avoid hidden costs.
January 28 2026
Future Role of Authorized Generics: Market Outlook
Authorized generics let brand manufacturers sell their own drugs at lower prices after patent expiry. Learn how this strategy is evolving with new FDA rules, rising drug costs, and shifting market dynamics to impact patient access and pricing.
December 1 2025
Generic vs Brand Identification in Pharmacy Systems: Best Practices for Accurate Medication Management
Learn how pharmacy systems distinguish between generic and brand drugs using NDC codes, FDA therapeutic equivalence ratings, and real-time data. Discover best practices to prevent errors, improve patient safety, and reduce costs.
December 1 2025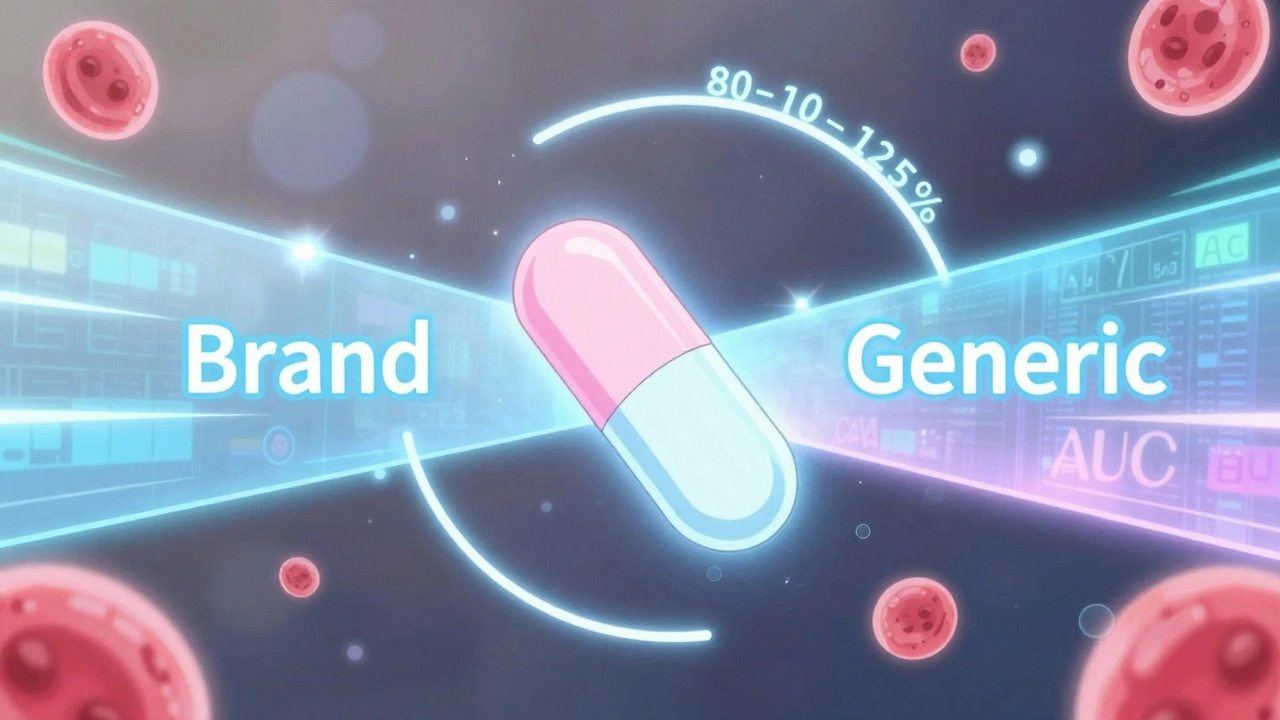
The 80-125% Rule: Understanding Bioequivalence Confidence Intervals for Generic Drugs
The 80-125% rule ensures generic drugs are absorbed the same way as brand-name versions. It's based on pharmacokinetic data, not drug content, and is used globally to approve safe, affordable generics.
December 1 2025
False Advertising in Generics: Legal Risks and Rules You Can't Ignore
False advertising in generic drugs misleads patients, endangers health, and violates federal law. Learn the legal risks, FDA rules, and real-world consequences of deceptive pharmaceutical marketing.
November 29 2025