Bioequivalence: What It Means for Generic Drugs and Your Health
When you switch from a brand-name drug to a generic, bioequivalence, the measure that proves two drug versions deliver the same amount of active ingredient at the same rate. Also known as therapeutic equivalence, it's the reason most generics are safe and effective replacements. This isn’t just paperwork—it’s science. The FDA requires generics to match the brand-name drug in how quickly and completely your body absorbs the medicine. If the bioequivalence numbers don’t line up within strict limits, the generic can’t be sold.
But bioequivalence doesn’t mean every pill feels the same. The active ingredient must be identical, but fillers, coatings, and manufacturing methods can vary. That’s why some people report subtle differences—like a generic causing more stomach upset or taking slightly longer to kick in. These aren’t always about bioequivalence failing; sometimes, it’s your body reacting to an inactive ingredient. Still, if you notice changes after switching—like your blood pressure spiking, your depression worsening, or your seizures returning—you’re not imagining it. That’s why FDA approval, the process that certifies a generic meets strict bioequivalence standards matters, but so does your own monitoring.
Drug absorption, how fast and how much of the medicine enters your bloodstream is the core of bioequivalence. It’s measured through blood tests after taking the drug. The generic’s absorption rate must fall within 80% to 125% of the brand-name version. That range isn’t arbitrary—it’s based on decades of clinical data showing most people won’t notice a difference within those bounds. But for drugs with narrow therapeutic windows—like warfarin, lithium, or seizure meds—even small shifts can matter. That’s why switching to a generic isn’t always a one-size-fits-all move. Some patients need to stick with the brand, or at least track their response closely after switching.
And here’s what you won’t hear from pharmacies: bioequivalence doesn’t guarantee identical side effects. Two drugs can be bioequivalent but still cause different reactions because of how the inactive ingredients interact with your body. A generic version of a thyroid med might use a different dye that triggers a reaction in someone with sensitivities. That’s not a bioequivalence failure—it’s a formulation difference. That’s why knowing your own body matters more than any label.
The posts below cover real cases where bioequivalence played a role—whether it was someone whose blood levels dropped after switching generics, a patient who noticed new anxiety with a cheaper version of their antidepressant, or how the FDA handles complaints when people say their generic isn’t working like it used to. You’ll find guides on when to monitor your health after switching, how to report problems, and why some medications demand extra caution. This isn’t about fear—it’s about awareness. You have the right to know if your medicine is truly interchangeable, and how to tell if it’s working as it should.

How the FDA Ensures Generic Drug Safety Through Manufacturing Oversight
The FDA ensures generic drug safety through strict bioequivalence testing, mandatory manufacturing inspections, and ongoing surveillance. Generics must match brand-name drugs in strength, quality, and performance-backed by science, not just cost savings.
January 22 2026
Therapeutic Failures: When a Generic Drug Doesn't Work as Expected
Generic drugs are supposed to be safe and effective alternatives to brand-name medications-but sometimes they don’t work as expected. Learn why therapeutic failures happen, which drugs are most at risk, and what you can do to protect yourself.
January 16 2026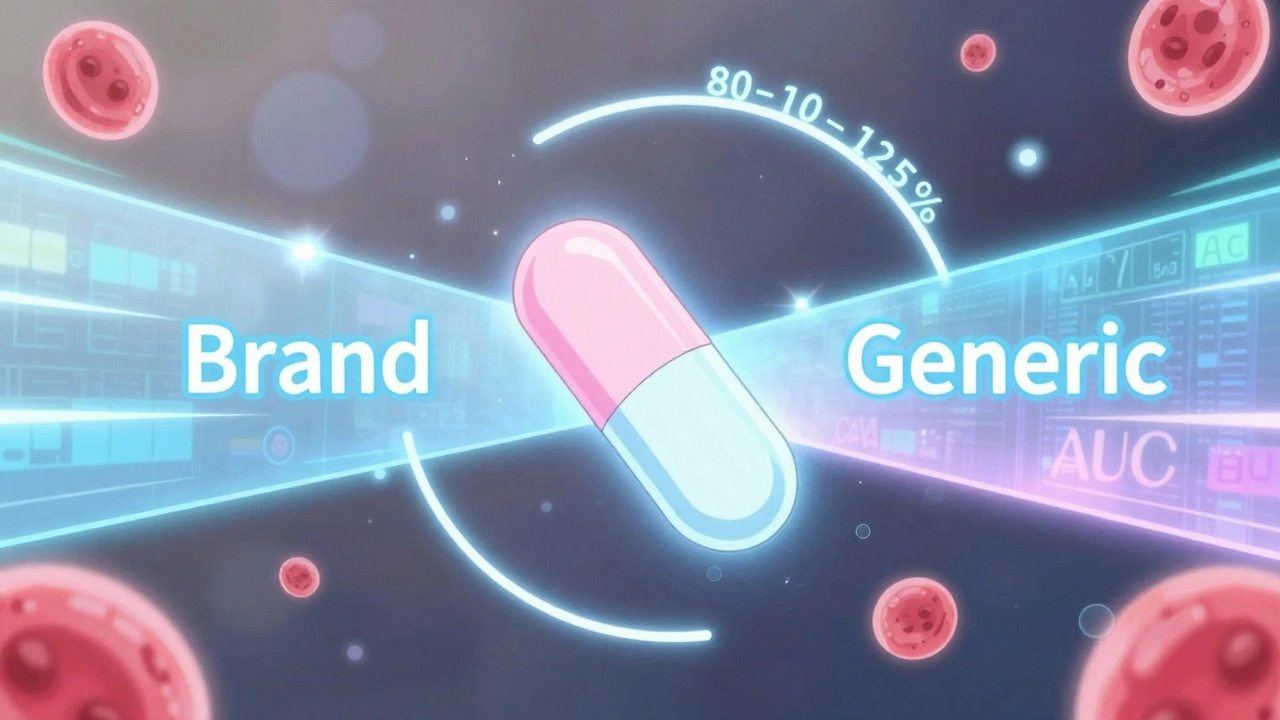
The 80-125% Rule: Understanding Bioequivalence Confidence Intervals for Generic Drugs
The 80-125% rule ensures generic drugs are absorbed the same way as brand-name versions. It's based on pharmacokinetic data, not drug content, and is used globally to approve safe, affordable generics.
December 1 2025